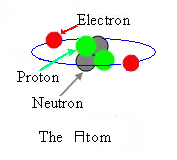
Introduction
Chapter 1 - Electricity
Chapter 1.2 - The Numbers
Chapter 2 – Sharing and Bonding
Chapter 3 - Voltage
Chapter 3.2 – Voltage Static
Chapter 3.3 - Batteries
Chapter 3.4 – Solar - Others
Chapter 4 - Resistance
Chapter 4.2 – Parallel Resistance
Chapter 4.3 – Voltage Dividers
Chapter 5 - Semiconductor
Chapter 5.2 - PNP NPN Junctions
Chapter 6 – AC and Hertz
Chapter 7 - Magnetism
Chapter 7.2 - Inductors
Chapter 8 - Capacitor
Chapter 9 - IC's and Amplifier
Chapter 10 - 555 Timer
Chapter 11 - Logic
Chapter 12 - Power Supply
|
|
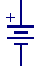 This is the schematic symbol for a battery. Take a moment and follow
this link to check out
This is the schematic symbol for a battery. Take a moment and follow
this link to check out 
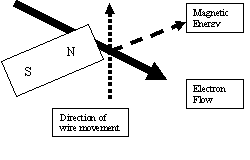 The index finger represents the direction of the magnetic energy coming out of the
north pole of the magnet. The thumb indicates the direction, the conductor (wire)
is moving along the face of the magnetic field. The middle finger represents both
the conductor and points in the direction the electrons are flowing along the
conductor.
The index finger represents the direction of the magnetic energy coming out of the
north pole of the magnet. The thumb indicates the direction, the conductor (wire)
is moving along the face of the magnetic field. The middle finger represents both
the conductor and points in the direction the electrons are flowing along the
conductor.
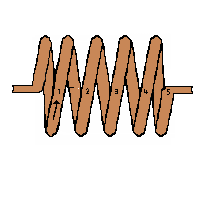 If we had a conductor with 5 loops of wire in a coil the result would produce
5 times more voltage than is produced in the single loop example. A 300-loop
coil would produce about 300 times the voltage.
If we had a conductor with 5 loops of wire in a coil the result would produce
5 times more voltage than is produced in the single loop example. A 300-loop
coil would produce about 300 times the voltage.
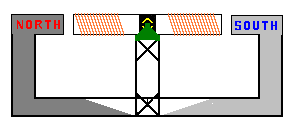 Many coils of wire are wrapped around an armature
(center metal component) of the generator. The north and south poles are part of
the stationary or stator section of the generator.
Many coils of wire are wrapped around an armature
(center metal component) of the generator. The north and south poles are part of
the stationary or stator section of the generator.