Introduction
Chapter 1 - Electricity
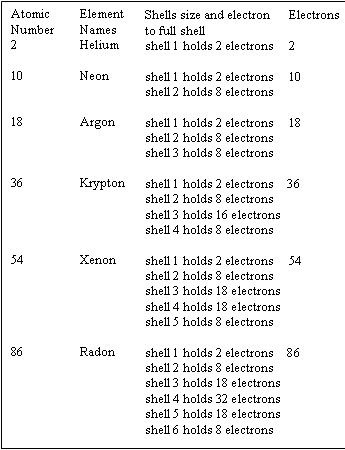
Chapter 1.2 - The Numbers
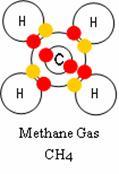
Chapter 2 – Sharing and Bonding
Chapter 3 - Voltage
Chapter 3.2 – Voltage Static
Chapter 3.3 - Batteries
Chapter 3.4 – Solar - Others
Chapter 4 - Resistance
Chapter 4.2 – Parallel Resistance
Chapter 4.3 – Voltage Dividers
Chapter 5 - Semiconductor
Chapter 5.2 - PNP NPN Junctions
Chapter 6 – AC and Hertz
Chapter 7 - Magnetism
Chapter 7.2 - Inductors
Chapter 8 - Capacitor
Chapter 9 - IC's and Amplifier
Chapter 10 - 555 Timer
Chapter 11 - Logic
Chapter 12 - Power Supply
|
|
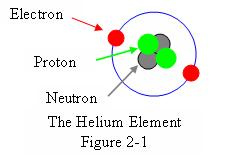 Here the outermost shell, or Valence Shell, in this case the only shell,
has two electrons in orbit. This is the maximum number of electrons that this shell
can hold, so the shell is full.
Here the outermost shell, or Valence Shell, in this case the only shell,
has two electrons in orbit. This is the maximum number of electrons that this shell
can hold, so the shell is full.